Early risk assessment is crucial to developing effective therapies for the more than 6 million Americans who have Alzheimer’s disease. Jingwen Yan, Ph.D. (above), director of the Luddy School’s Bioinformatics program, recently has received a $1.9 million National Institutes of Health grant to more deeply explore the development of Alzheimer’s disease (AD).
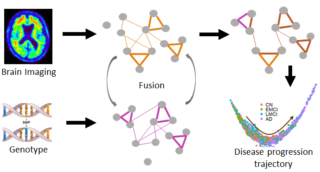
“In this project, we will leverage major multi-omic genetic data and multi-modal brain imaging data of Alzheimer’s disease (AD),” Yan says, “and build interpretable deep learning models to capture the continuous process of AD progression and the associated changes in brain imaging and multi-omics.”
“Our new algorithm will potentially capture the subtypes of progression, providing valuable information to facilitate improved precision medicine,” adds Yan, associate professor of Bioinformatics at IU’s Luddy School of Informatics, Computing, and Engineering in Indianapolis.
In September, Yan received the 5-year R01 grant through the NIH, a part of the U.S. Department of Health and Human Services, for her research project, “Characterizing the progression of Alzheimer’s disease with multi-omic genetic and imaging data.”
Yan also recently has had a paper published in a prestigious journal, related to her work with progression profiling of Alzheimer’s disease. She is the corresponding author of “Integrating amyloid imaging and genetics for early risk stratification of Alzheimer’s disease,” published in the September 2024 edition of Alzheimer’s and Dementia, the journal of the Alzheimer’s Association.
The research paper focuses on how integration of genetic data with brain scans that detect amyloid protein deposits can aid in assessing the risk of cognitive decline and progression into Alzheimer’s in the early stage. This is of great clinical value as therapeutic intervention is expected to be more effective when started early in the disease stage before evident symptoms.
Highly regarded research
“Modeling the progression of Alzheimer’s disease is one of the most promising approaches to the early detection and treatment of the disease,” says Shiaofen Fang, Ph.D., associate dean for research at Luddy Indianapolis.
“Professor Yan and her team have made significant progress in establishing omics biomarkers for AD progression as reported by their recent paper in Alzheimer’s and Dementia, the top journal in this field.
“This new R01 grant further indicates the research community’s confidence and recognition of her approach and research strength,” adds Fang, a professor of computer science at the Luddy School.
R01 grant focuses on Alzheimer’s biomarkers
“Alzheimer’s disease is an irreversible neurodegenerative disorder with a long prodromal phase and no clinically validated cure,” Yan writes in the abstract for her R01 research project.
“Early biomarkers are believed crucial to AD, making it possible to identify and treat AD patients before evident symptoms.
“In this project, we aim to develop novel computational approaches to identify genetic biomarkers related to AD progression and possible subtypes.”
Genetic biomarkers: A possible point of attack
Discovering specific genetic biomarkers for conditions such as Alzheimer’s is an important step toward developing targeted treatments, and the purpose of Yan’s NIH research project. It has the potential to make a real difference for those at risk of developing AD.
“These results can help with candidate screening in clinical trials and provide stratified risk groups to facilitate the development of targeted therapeutic intervention,” Yan writes.
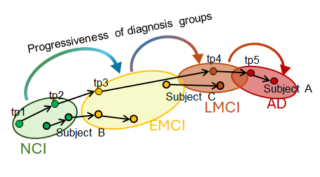
“Detecting when and how molecular and phenotype markers develop along AD progression will provide a template” for understanding the process of AD development, Yan explains, “and for improving early diagnosis, clinical trial recruitment and treatment assessment.”
The research project has the potential to help identify people with Alzheimer’s who would be most likely to benefit from new treatments.
“The proposed methods and tools will have considerable potential for discovery of omics biomarkers to help monitor the disease progression,” Yan says, “and provide insights of downstream molecular mechanism toward the development of Alzheimer’s disease.
“Our capability to subtype patients could help with candidate screening in clinical trials and provide stratified risk groups to facilitate the development of therapeutic intervention.”
About the NIH R01 grant
The Research Project Grant (R01) is the original and historically oldest grant mechanism used by NIH. The R01 provides support for health-related research and development based on the mission of the NIH. Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award number 1R01AG081951-01A1. Total program or project costs of $1.9 million are financed with federal money. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Media Contact
Joanne Lovrinic
jebehele@iu.edu
317-278-9208


